Topic outline
-
-
The Concept of Matter
Matter is the name given to all the things around you. Matter is any substance which has mass and occupies space. All physical objects are composed of matter, in the form of atoms and molecules, which are in turn composed of protons, neutrons, and electrons.
All matter can be categorised as a solid, liquid, gas, or a plasma. This is called the states of matter.
Classification of Matter
1: Pure Substance: Matter with a consistent arrangement.
Element: comprised of just one kind of atom. Example - gold, silver, carbon, oxygen, and hydrogen
Compound: at least two different elements which are chemically joined together. For example - water, carbon dioxide, sodium bicarbonate, carbon monoxide
2: Mixed Substance: matter with a variable creation.
Heterogenous mixtures: A heterogeneous mixture is one that contains two or more substances in a different state. The substances in the mixture are not evenly distributed throughout the mixture. For example, sand and soil.
Homogeneous Mixtures: A homogeneous mixture can be a gaseous, liquid, or solid mixture that has the same proportions of its components throughout a given sample. There is only one phase of matter observed in a homogeneous mixture. For example - saltwater.
Homogenous mixtures can be further classified as:
Colloids: Colloids are mixtures in which microscopically dispersed insoluble particles of one substance are suspended in another substance. For example, flour and water.
Solutions: In a solution, all the components appear as a single phase and the particles are evenly distributed. This is why a whole bottle of soft drink has the same taste throughout.
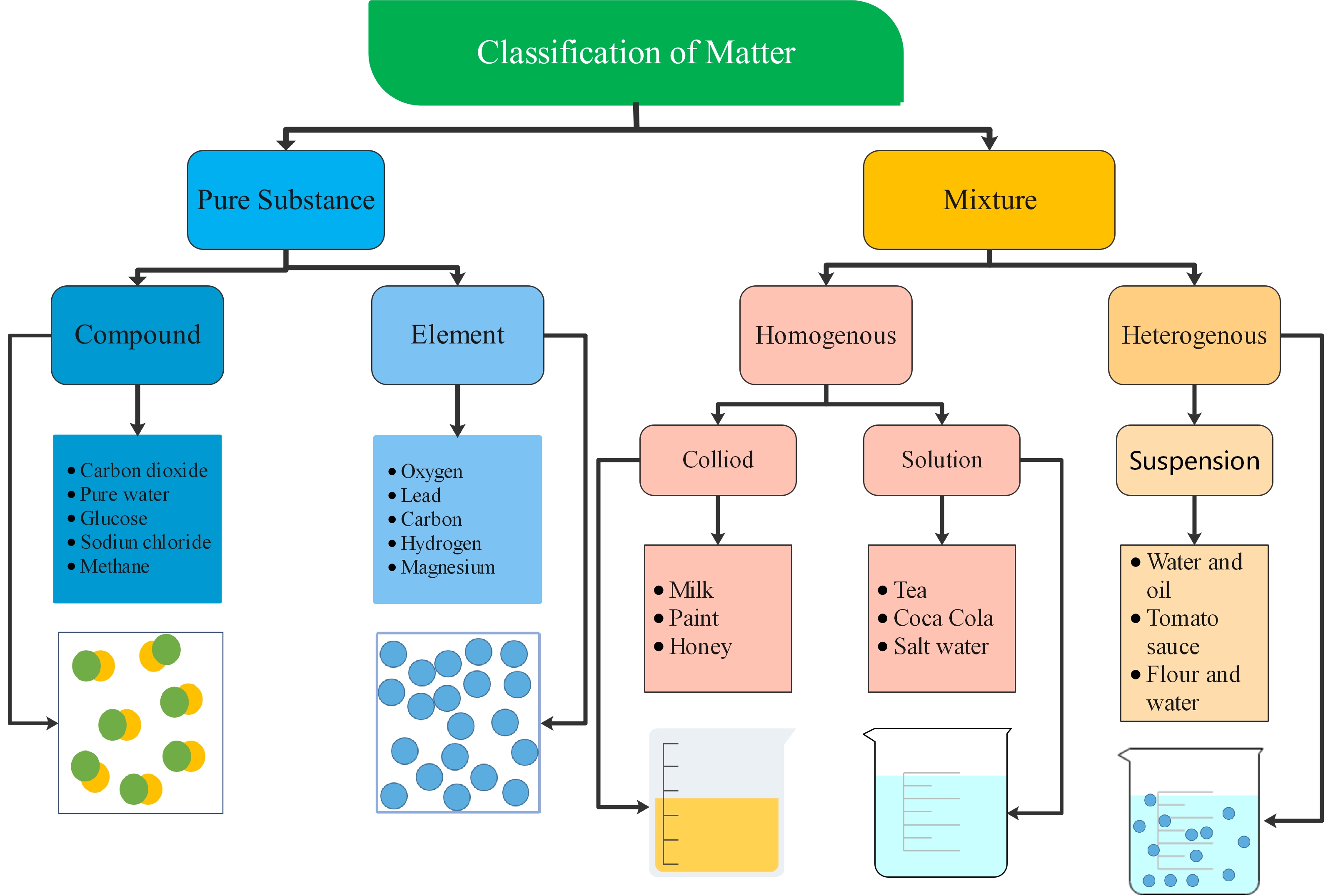
Figure 1: A concept map of the classification of matter.Characteristics of Particle Nature of Matter
The following are the characteristics of the particle nature of matter:
- Atoms and molecules are tiny particles. They are not visible to the naked eye.
- Particles always attract each other: The molecules in solids have no space as they are closely bonded by a strong force of attraction; this force is weaker in liquid and gas.
- All particles are continuously in motion. When the particles are heated, their speed changes and they begin to move quickly. The opposite is true when particles cool.
- There is space between the particles of matter. When sugar is dissolved into water, the sugar crystals separate into very fine particles. These particles of sugar occupy the spaces between the various particles of water. Hence, there is no change in the volume of water.
Brownian Motion
Activity 1
1. Place a few crystals of potassium permanganate in a glass of water. If potassium permanganate is not available, use a couple of drops of food colouring.
2. Observe.
3. Heat some water and repeat step 1. What do you notice about speed at which the potassium permanganate moves through the water?

Figure 2: The 'spreading out' of potassium permanganate in a beaker of water.
Conclusion
When a potassium permanganate crystal is placed in a water beaker, the water slowly turns purple on its own, without stirring. The particles separate each other and spread throughout the water turning the water completely purple. When the water temperature is increased, the particles spread out through the water more quickly.
What is actually happening is on dissolving, the particles of potassium permanganate get into the spaces between the particles of water.
So, it can be concluded that the particles are moving, or they are in motion.
Brownian motion refers to the random movement displayed by small particles that are suspended in fluids. This motion is a result of the collisions of the particles with other fast-moving particles in the fluid.
Brownian motion is named after the Scottish Botanist Robert Brown, who first observed that pollen grains move in random directions when placed in water.
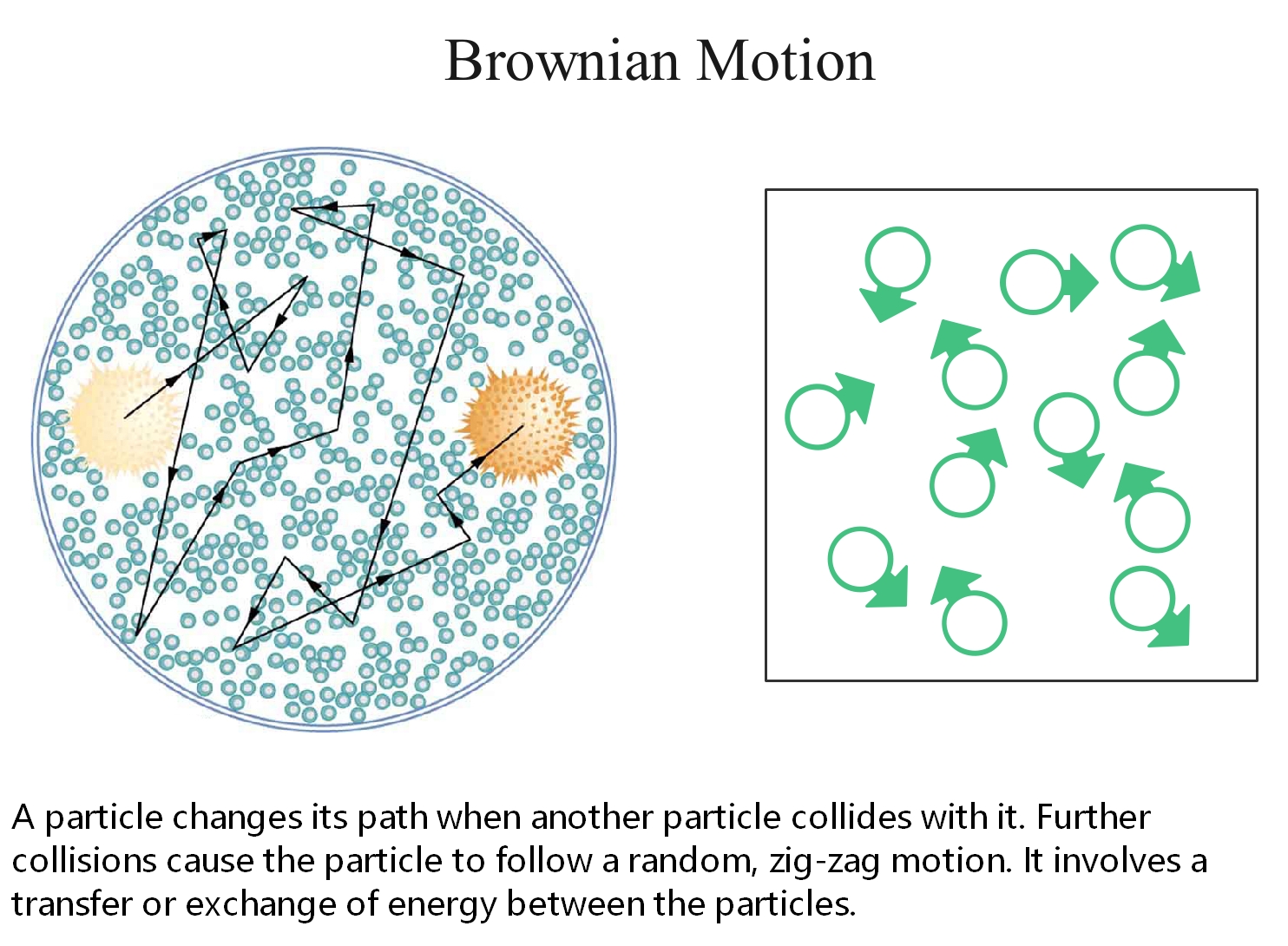
Figure 3: An illustration describing the random movement of fluid particles caused by the collisions between these particles.
-
-
-
The kinetic particle theory explains the properties of solids, liquids and gases. The work kinetic means movement.
All matter consists of particles including atoms and molecules. In everyday life, there are three states of matter - solids, liquids, and gases. The differences between the three states are due to the arrangement and spacing of the particles and their motion.
Kinetic theory of matter states that “Matter is made up of those substances or particles which are constantly moving.” The energy level of the particles depends upon the temperature possessed by the matter. This helps us to determine whether that matter is in a solid, liquid, or gas state.
Activity 1: Make a model to see how particles behave
What you will need:
· a rectangular box (as big as a shoebox lid,) large enough to allow free movement for five marbles
· 5 marbles (or small hard balls of the same size) – one should be different in colour from the others
· a broad flat board that can fit into the box and can close off the end of the box (see diagram in Part A), like a small ruler
· a watch or timer, for example on a cellphone
Task 1
Place the marbles in the box on a flat surface such as a table. (Remember that your box should be large enough to allow free movement of the marbles.) Shake the box in all directions for 30 seconds, but without lifting it off the table. Then answer the questions below:
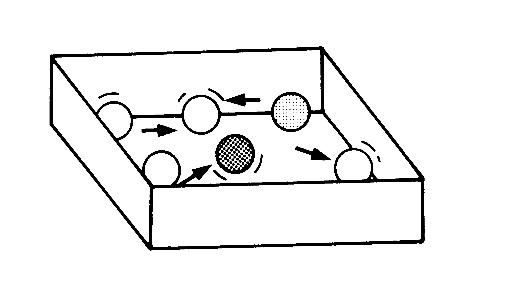
1. Describe the movement of the marbles.
2. Can you predict where a marble will move? Explain your answer.
3. Repeat this task and watch one of the marbles. List all the things it bumps against in the 30 seconds.
We call this disorganised movement shown by the marbles random motion.
In task 2, we will reduce the space in the box to find out how the marbles behave in a smaller space.
Task 2
Use the broad flat board (or a ruler) to make the space inside the box half as big, as shown here. Now shake the box as before. Answer the following questions:
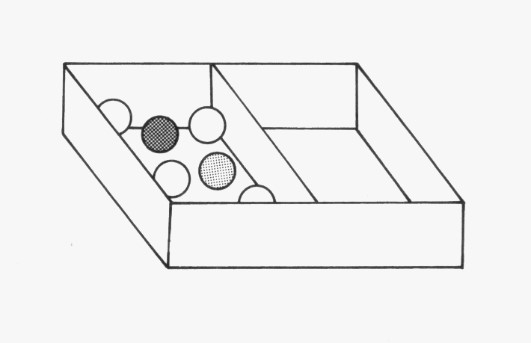
1. Compare the movement of the marbles with your observations in task 1.
2. Count the number of collisions the marble makes with its neighbouring marbles and with the sides of the box.
In task 3 we will decrease the space even further.
Task 3
Place your board such that the marbles are as tightly packed as possible, but do not squash them. Shake the box as before, and then answer the questions below:
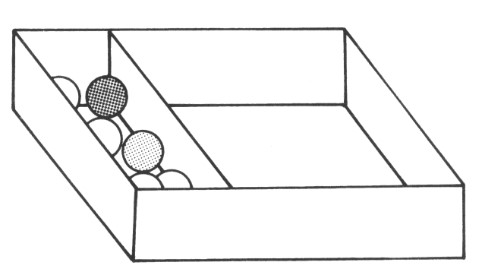
1. How do the marbles move now?
2. Shake the box faster.
3. Can you see any difference in the motion of the marbles?
4. Count the collisions of the marbles and compare the movement of the marbles now to how they moved in tasks 1 and 2 of this activity.
What did you find?
In task 1, the marbles were far apart and moved freely in a large space. This is also true of the particles in a gas. They have large spaces between them and move about freely. You would have observed, during the activity, that the particles collide with one other and with the sides of the container. There are also large spaces between the particles, on average. By 'on average' we mean there are large spaces most of the time except when they are colliding. When they are colliding, they are close together. But they have great freedom of movement.
In task 2, the particles were closer together but very loosely packed and there was some room for the marbles to move. This is like the arrangement of particles in a liquid, where the particles are close together, but they can still slide over one another.
In task 3, the marbles were fixed in position and could not move about. This arrangement is like the arrangement of particles in solids.
-
-
-
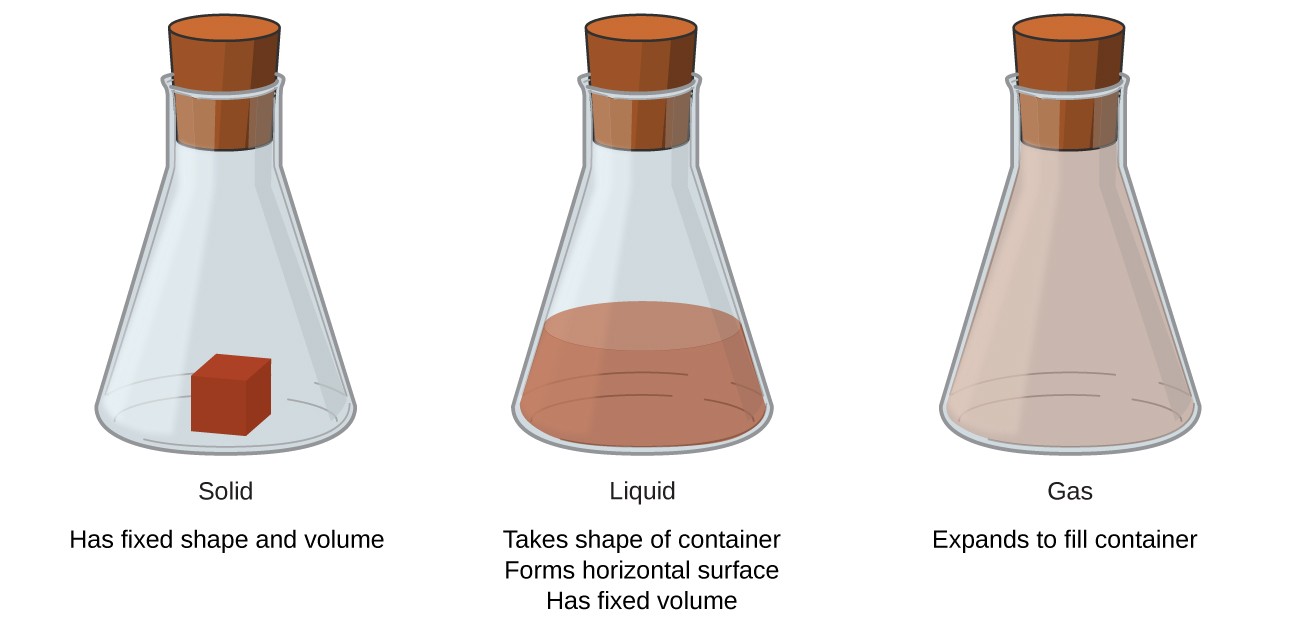
States of matter refers to the different forms in which a matter can exist. In broader terms, there are four different states of matter namely solid, liquid, gas, and plasma. Matter in different states exhibit distinct physical and chemical properties. While a matter in a solid has a fixed volume and shape, it loses its shape in liquid form, but its volume remains the same. In gaseous state, both the volume and shape are not fixed.
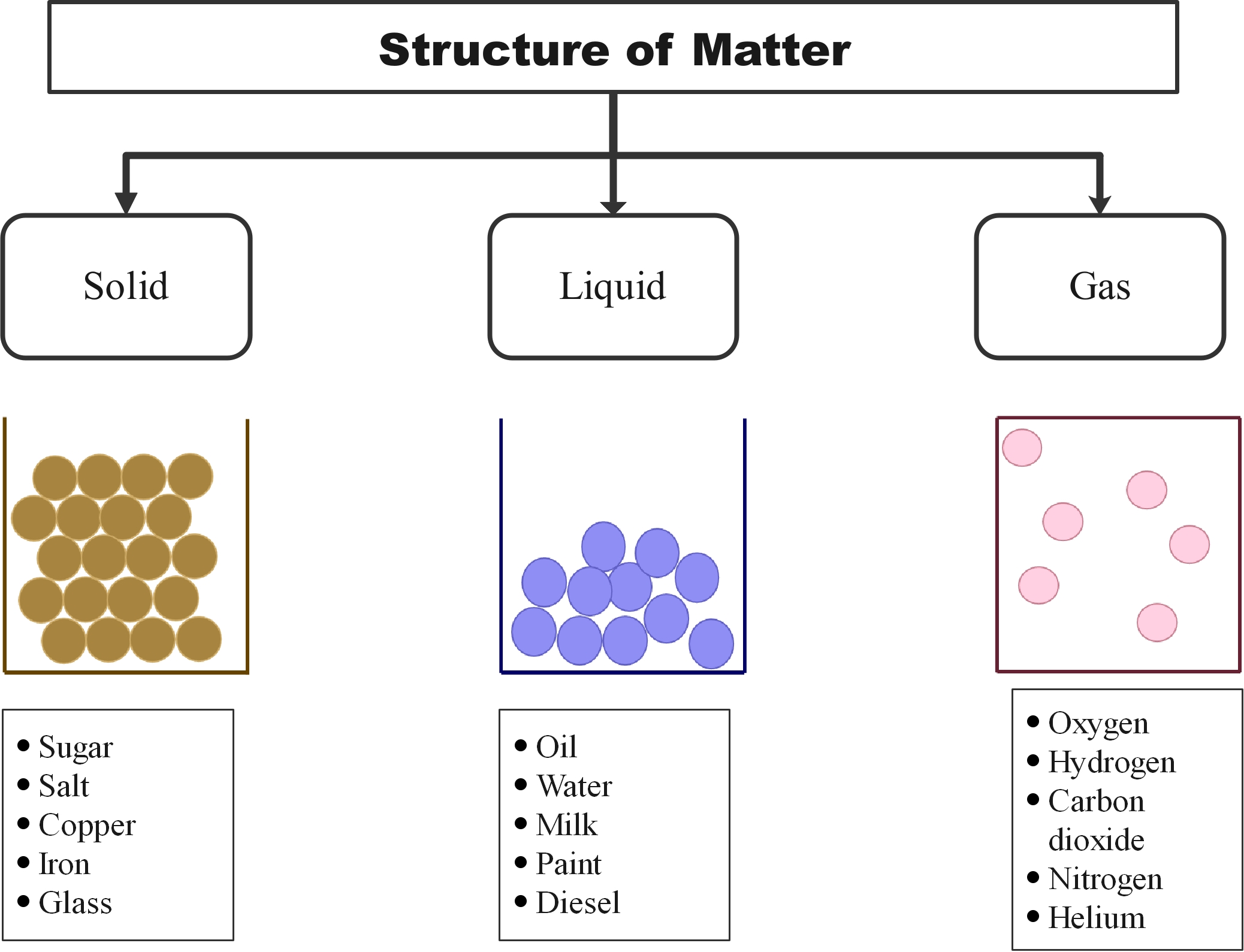
Figure 1: The three states of matter
Solids
The particles in a solid:
- sit very closely together.
- are in a regular arrangement and in fixed position.
- vibrate about a fixed position but do not move through the solid.
- are held together by strong forces.
This explains why solids have a fixed shape and volume.
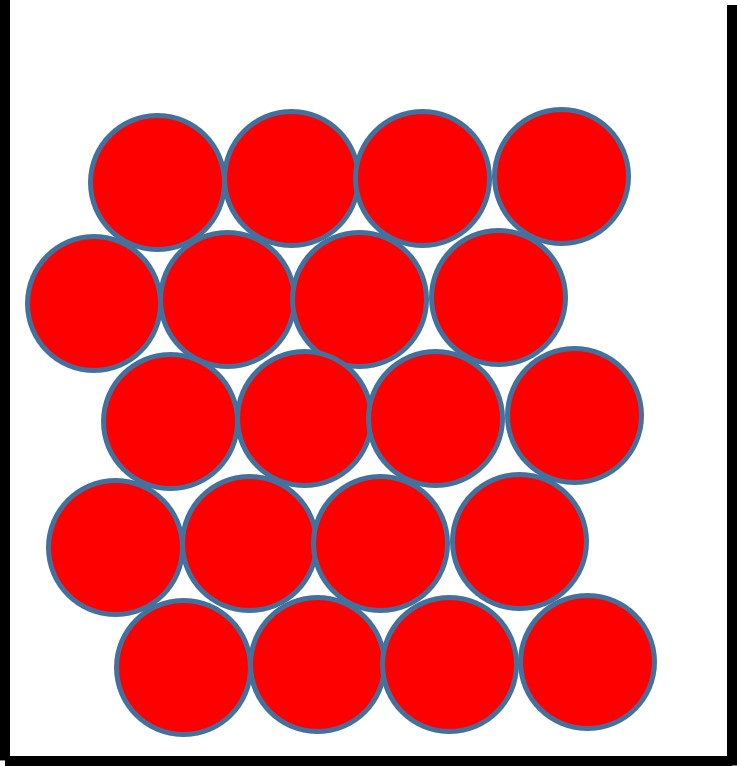
Figure 2: the particle arrangement in a solid
Liquids
The particles in a liquid:
- sit close together but have some gaps.
- can move past each other because of the gaps.
- have enough energy to prevent the forces between them holding them in a fixed, regular arrangement.
- are randomly arranged.
This explains why liquids have a fixed volume but take on the shape of their container.
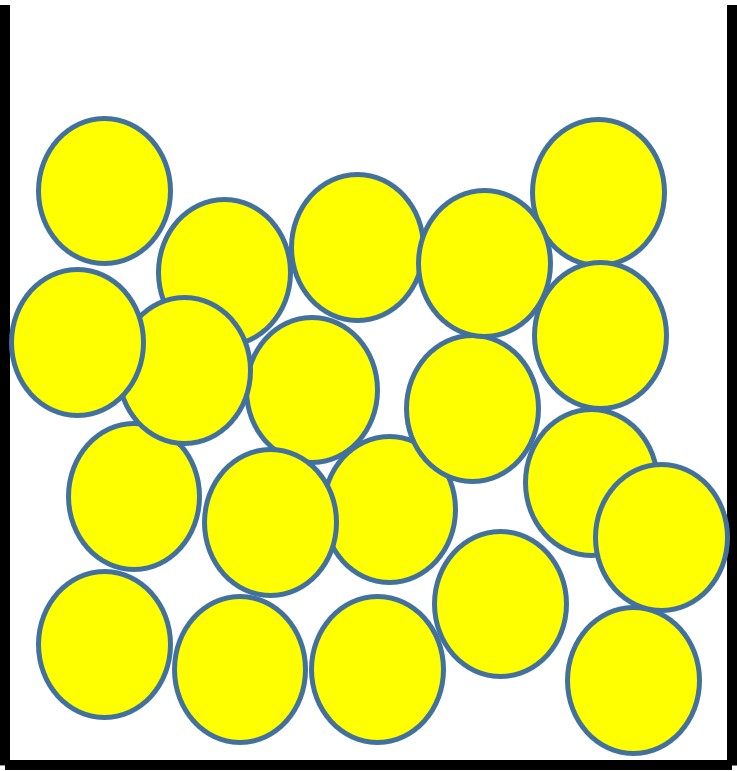
Figure 3: the particle arrangement in a liquid
GasesThe particles in a gas:
- are much further apart than in a solid or liquid.
- are entirely free to move because the forces between them are weak.
- are randomly arranged.
- move quickly and randomly in all directions.
This explains why gases completely fill their container and have the same volume as their container.
Figure 4: the particle arrangement of a gas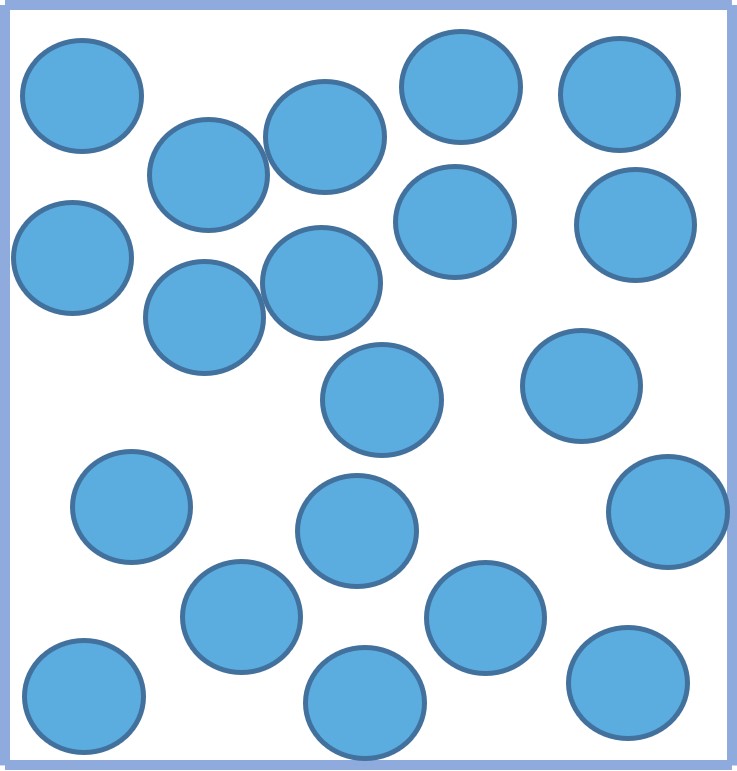
-