Topic outline
-
-
-
One of the fundamental quantities in science is temperature, which is a measure of the average amount of kinetic energy, a system contains.
When the temperature increases, the motion of these particles also increases. In other words, temperature determines the internal energy within a given system.
Temperature is measured with a thermometer or a calorimeter. Temperatures are expressed using scales that use units called degrees.
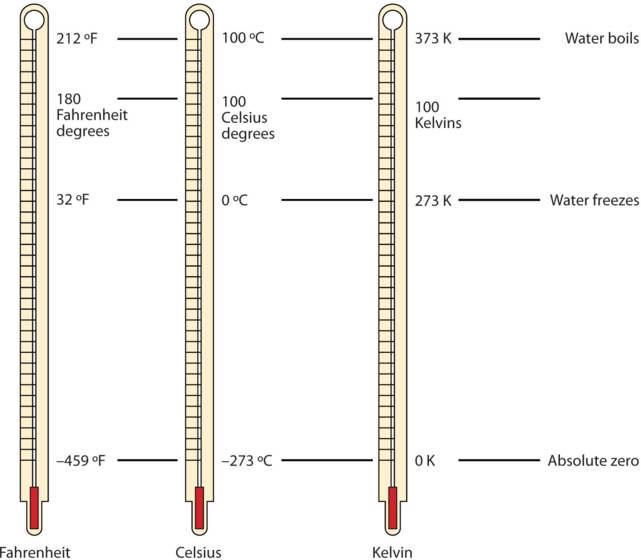
Figure 1: There are three scales used for reporting temperatures.
The Celsius scale (symbolised by °C and spoken as “degrees Celsius”) defines 0°C as the freezing point of water and 100 °C as the boiling point of water.
This scale is divided into 100 divisions between these two landmarks and extended higher and lower as well.
Note that science uses the Celsius and Kelvin scales. The United States is one of the few countries in the world that still uses the Fahrenheit scale daily.
The SI unit for temperature is the Kelvin (K).
The Kelvin temperature scale uses degrees that are the same size as the Celsius degree, but the numerical scale is shifted up by 273.15 units. That is, the conversion between the Kelvin and Celsius scales is as follows:
K=0C+273.15
For example: 160C in Kelvin = 16 + 273 = 289K
For most purposes, it is acceptable to use 273 instead of 273.15.
Note that the Kelvin scale does not use the word degrees; a temperature of 295 K is spoken of as “two hundred ninety-five kelvin” and not “two hundred ninety-five degrees Kelvin.”
The reason that the Kelvin scale is defined this way is that there exists a minimum possible temperature called absolute zero (zero Kelvin). The Kelvin temperature scale is set so that 0 K is absolute zero, and the temperature is counted upward from there.
Heat vs Temperature
It is important to understand that heat and temperature are not the same. Although the two concepts are linked, they mean different things.
Heat describes the transfer of thermal energy between molecules within a system and it is measured in joules. An object can gain or lose heat, but it cannot have heat. Heat is not a property possessed by an object or system rather it is a measure of change.
In conclusion, we can say that heat is a transfer of thermal energy caused by a difference in temperature between molecules.
-
-
-
Relationship Between Temperature and Kinetic Energy
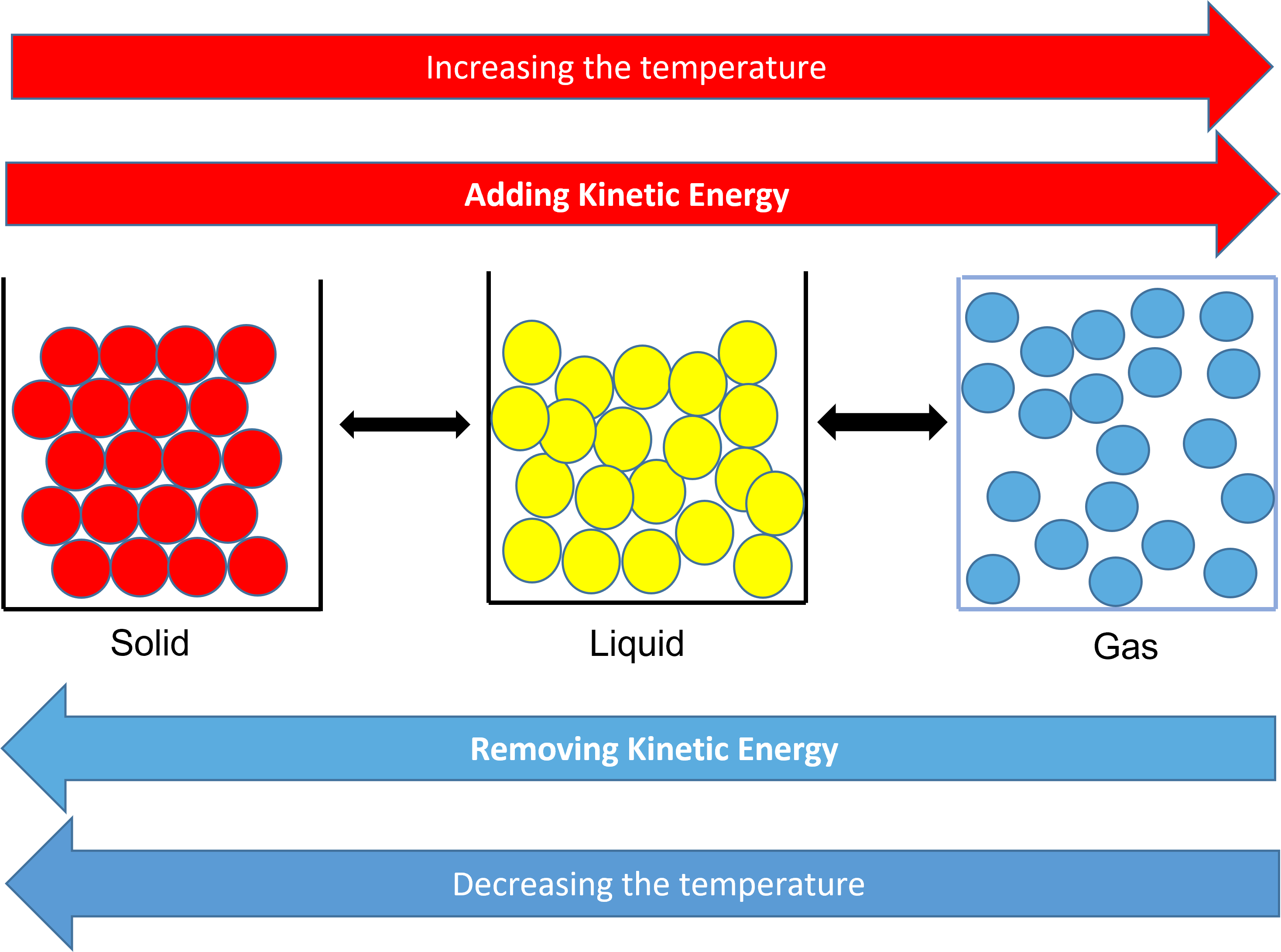
Kinetic energy is the energy possessed by a body due to its motion. We see a range of kinetic energy in molecules because all molecules don’t move at the same speed. When a substance absorbs heat, the particles move faster, so the average kinetic energy and therefore the temperature increases.
As stated in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, energy increases the motion of the particles. This will cause an increase in the temperature of the substance.
Solids, liquids, and gases all have a temperature. The particles within a solid don’t move, but they vibrate. The temperature increases when molecules vibrate faster. The melting point of a solid is the temperature at which the vibrational motion overcomes the forces of attraction holding the molecules in a solid formation. This is when it changes state. A change of state is a physical property.
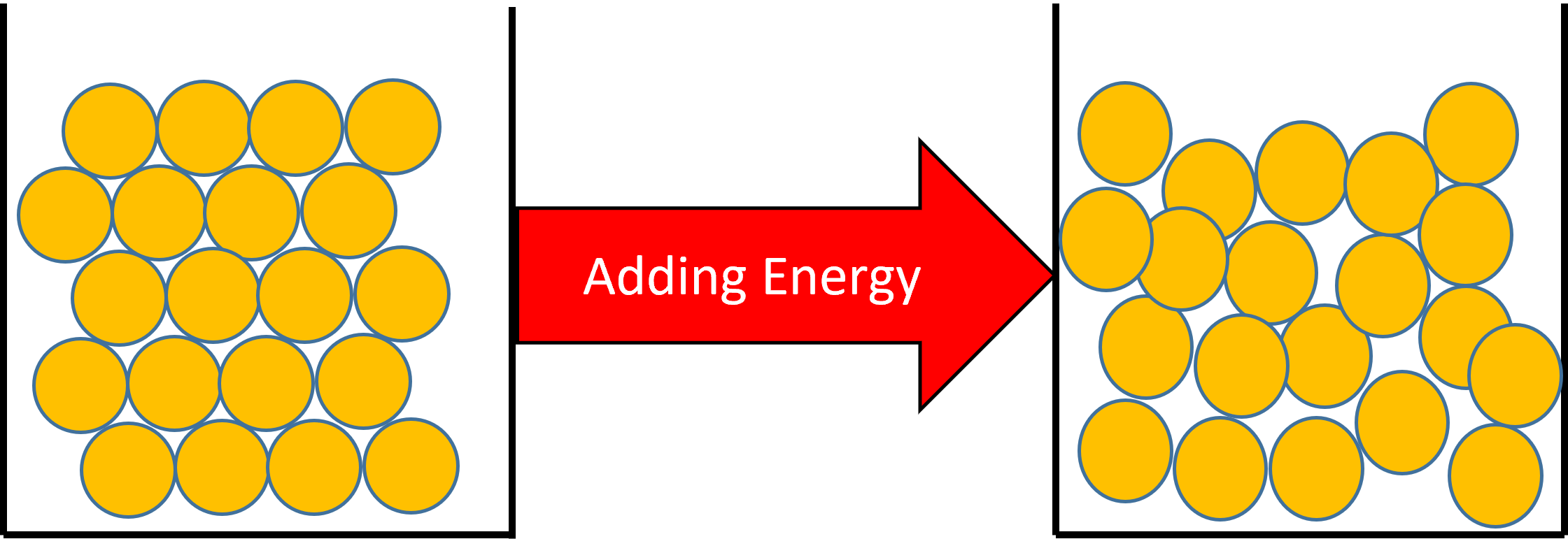
Figure 1: When you add heat to a solid, the particle's kinetic energy will increase. If enough energy is added, the particles in the solid will have enough energy to change their arrangement and become a liquid.
A change of state from a solid to a liquid will have the effect of increasing the substances temperature.
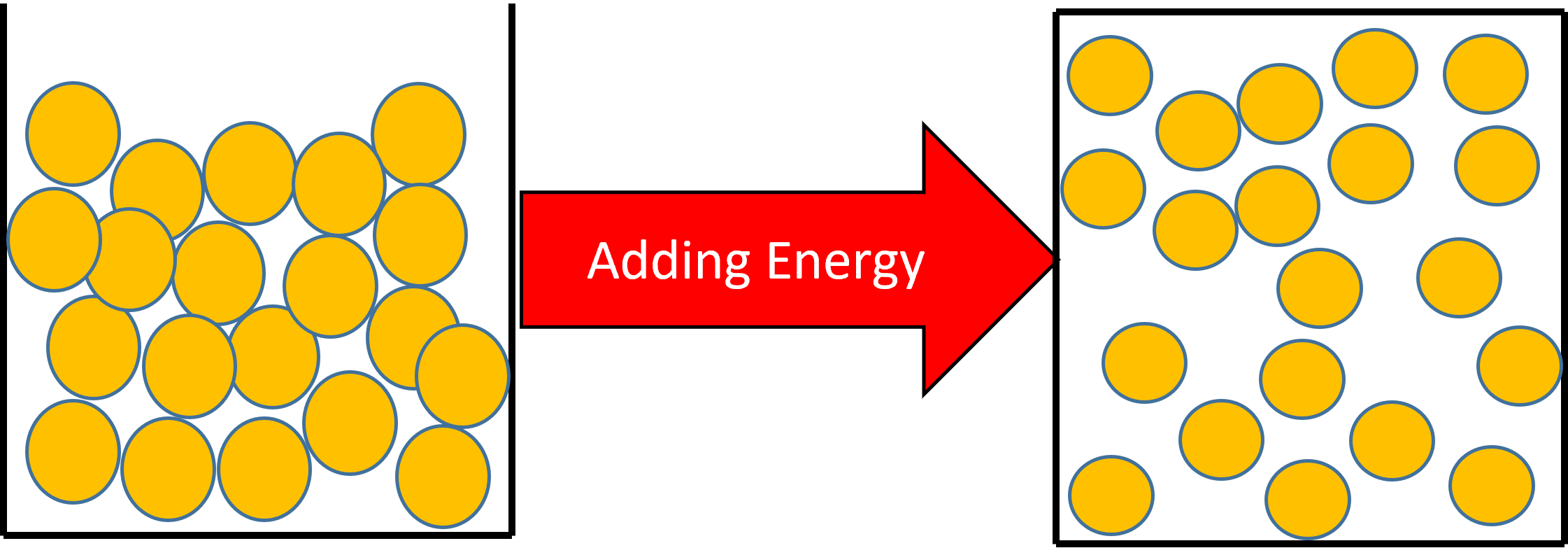
Figure 2: If you continue heating the liquid, the particles will gain more kinetic energy. If enough energy is added, the particles will have enough kinetic energy to change their arrangement and become a gas.
A change of state from a liquid to a gas will have the effect of increasing the substances temperature.
Activity 1
1. Click on the link below:
https://phet.colorado.edu/sims/html/states-of-matter-basics/latest/states-of-matter-basics_en.html
On the home screen press the states tab.
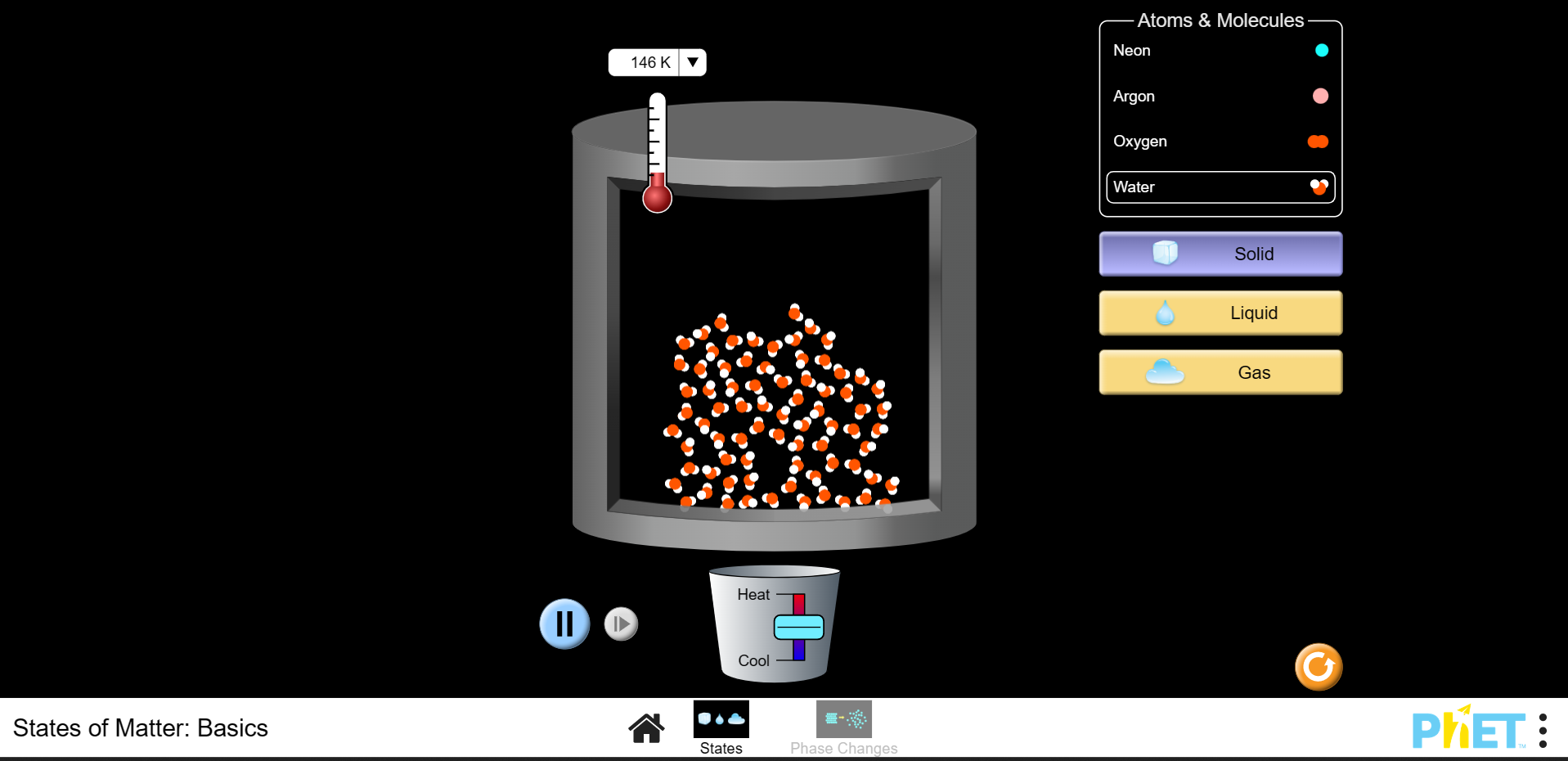
2. Using the menu on the right-hand side, press water icon and the solid icon. What do you notice about the behaviour of the particles?
3. Change the temperature from Kelvin to Celsius. Using the lever on the bucket under the molecules, increase the temperature until it reaches 00 C. What do you notice about the behaviour of the particles?
4. Using the lever, raise the temperature to 1000 C. What do you notice about the behaviour of the particles?
5. Continue to increase the temperature until it reaches approximately 2000C. Has the behaviour of the particles changed?
6. Using the lever, decrease the temperature and observe the changes in behaviour.
7. Reduce the temperature to the lowest possible temperature this should be somewhere close to 274K. How is the behaviour different than when the temperature was close to 00C?
Conclusion
As you increase the temperature, the particles gain more kinetic energy. This allows them to change state.
When you reduce the temperature, the particles loose kinetic energy and again they change state.
When water is near to absolute zero, the water particles, in the solid state of ice, do not even vibrate.
-
-
-
Temperature is measured with a thermometer. The basic operating principle behind all thermometers is that there is some quantity, called the thermometric variable that changes in response to changes in temperature. It is the thermometric variable that gets measured. There is no way to measure temperature directly.
Figure 1: The simplest of thermometers is the liquid thermometer. They are a thin glass tube filled with a small amount of alcohol usually coloured red.
Thermometers measure the temperature due to thermal expansion. An increase in the volume of the substance because of the increase in the temperature is known as thermal expansion. A slight change in the temperature causes changes in the volume of a liquid.
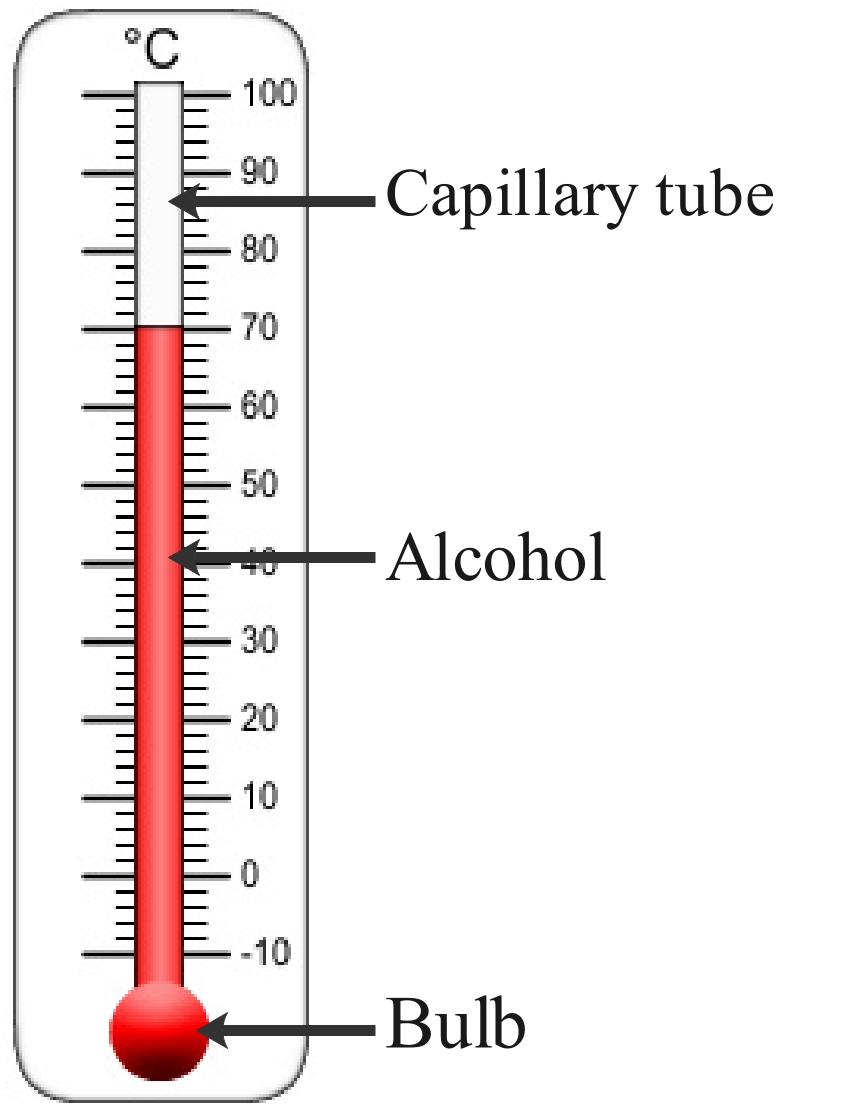
Figure 2: The alcohol in in the capillary tube expands when heated and contracts when cooled.
When heated, the molecules of the liquid in the thermometer move faster, causing them to get a little further apart. This results in movement up the thermometer. When cooled, the molecules of the liquid in the thermometer move slower, causing them to get a little closer together.
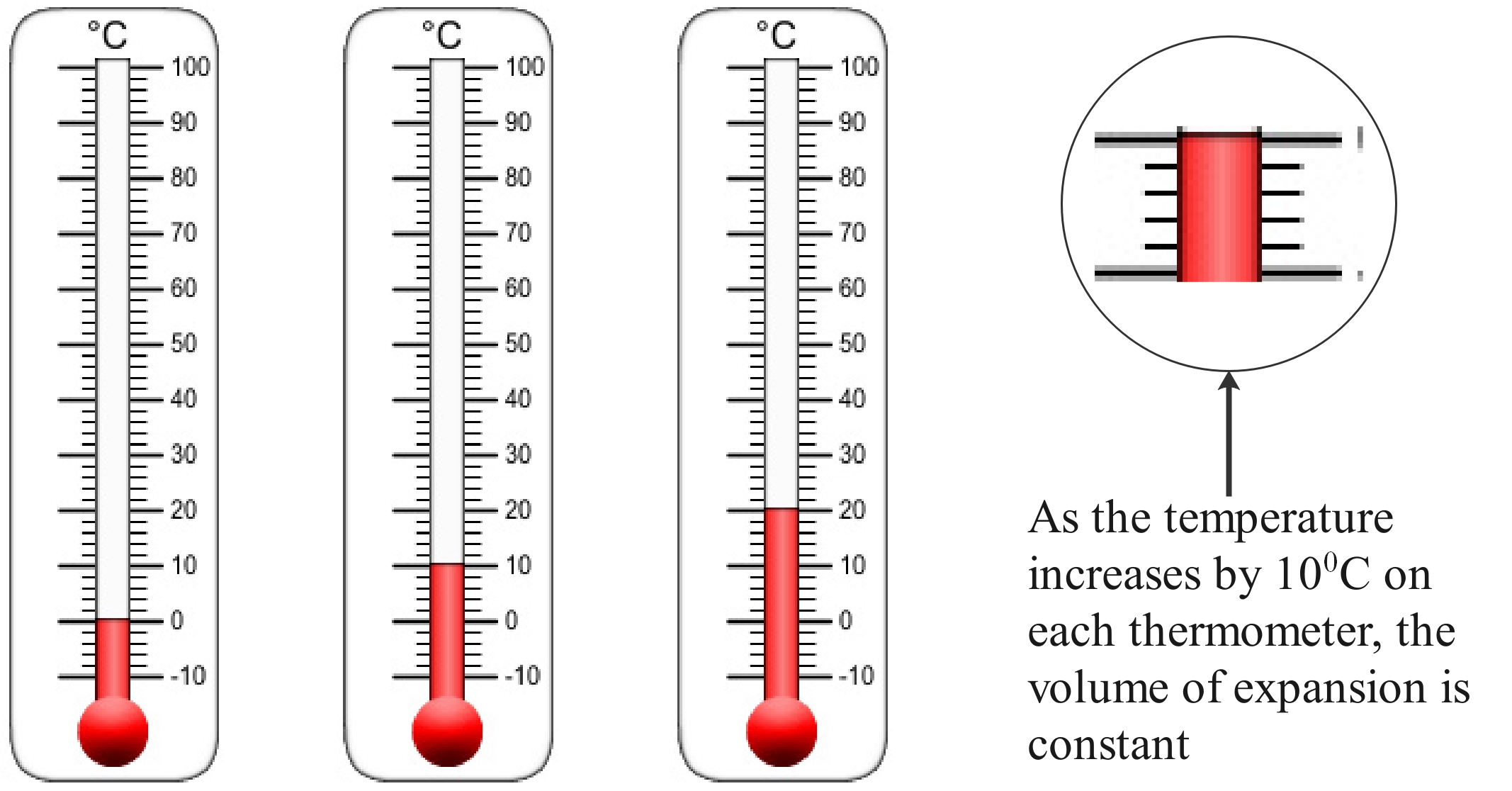
Figure 3: When the alcohol expands, it increases in size by an amount that’s directly related to the temperature.
If the temperature increases by 20 degrees, the alcohol expands and moves up the scale by twice as much as if the temperature increase is only 10 degrees.
The Celsius scale is based on the temperatures of ice and boiling water because these are the two fixed points. When we dip the thermometer into ice, we observe that the alcohol level marks 0°C on the scale. The alcohol rises to the top of the thermometer and reads 100°C if the thermometer is placed in boiling water.
Thermometers can also be filled with mercury, which behaves in the same way as alcohol in a thermometer. This is now no longer widespread practice because mercury is toxic.
-