Topic outline
-
-
-
Capillarity
Capillarity or capillary action is very important in moving water and other liquids around, usually against gravity.
Capillarity can be defined as the tendency of a liquid in a capillary tube or absorbent material to rise or fall because of surface tension.
Three main variables that determine whether a liquid possesses capillary action are:
- Cohesive force: It is the intermolecular bonding of a substance where the attractive forces cause them to maintain a certain shape of the liquid.
- Surface tension: This occurs because cohesive forces in molecules joining together to form a surface on the body of water.
- Adhesive force: When forces of attraction between unlike molecules happens.
Capillary action only occurs when the adhesive forces are stronger than the cohesive forces, which usually becomes surface tension, in the liquid.
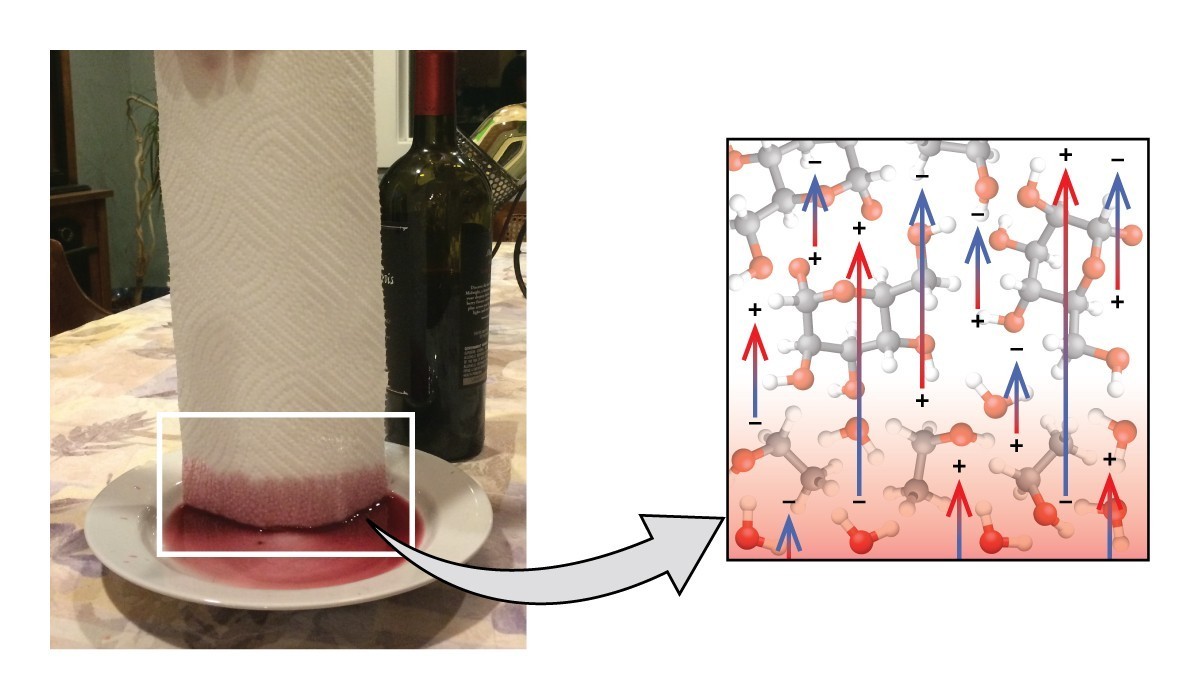
Figure 1: A liquid will travel up a paper towel (left) because of the strong attractions of water molecules to the towel’s cellulose fibers.
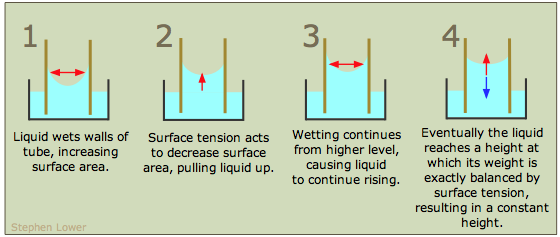
Figure 2: Capillary action can also occur when one end of a small diameter tube is immersed in a liquid.
If the liquid molecules are strongly attracted to the tube molecules, the liquid creeps up the inside of the tube until the weight of the liquid and the adhesive forces are in balance. The smaller the diameter of the tube is, the higher the liquid climbs.
It is partly by capillary action occurring in plant cells called xylem that water and dissolved nutrients are brought from the soil up through the roots and into a plant.
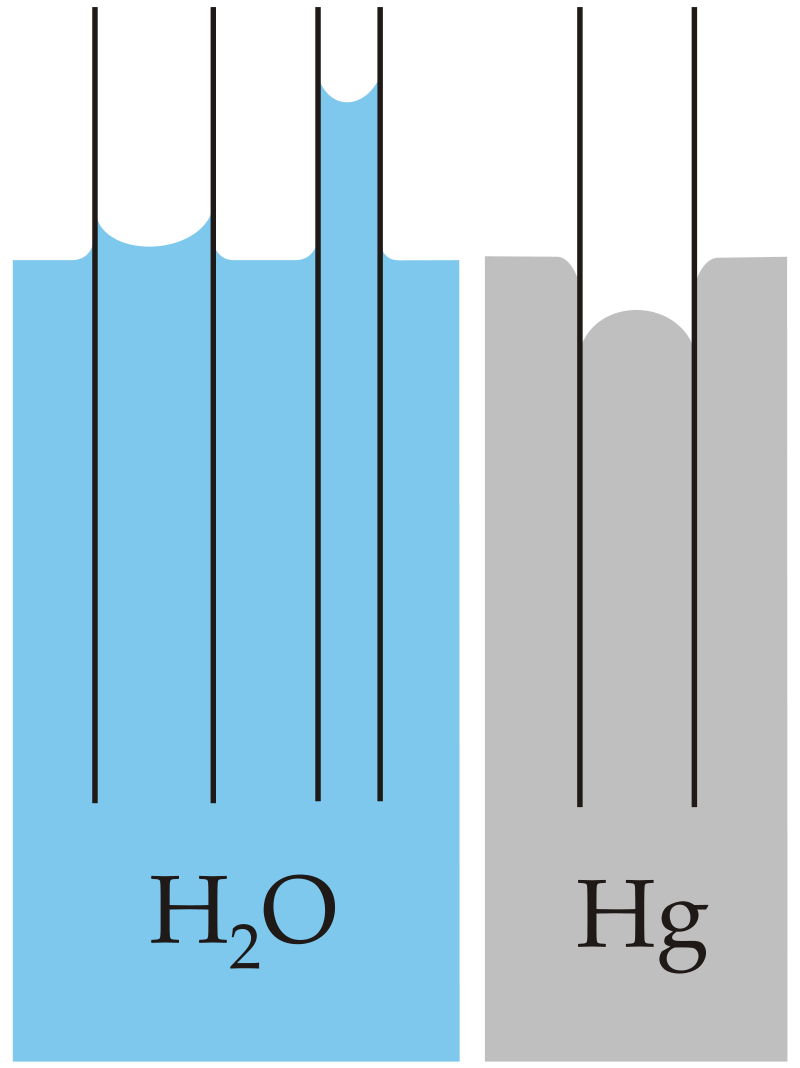
Figure 3: It is possible to see that in water, the strength of the adhesive forces is larger than the strength of the cohesive forces. This results in the concave formation of water in the capillary tube. This is known as capillary attraction.
For mercury, the cohesive forces are stronger than the adhesive forces which allows the meniscus to bend away from the walls of the capillary tube. This is known as capillary repulsion.
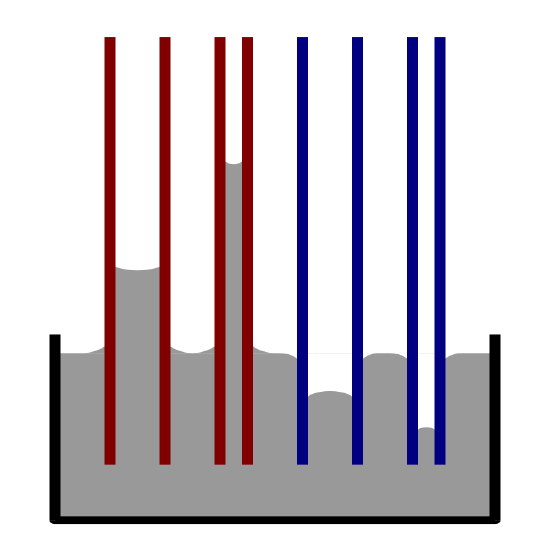
Figure 4: The diameter of the container as well as the gravitational forces will determine amount of liquid which will rise through the tube. The thinner the tube, the higher the liquid will rise.
Activity 1
What you will need:
3 glasses
3 pieces of paper towel
Food colouring -2 different colours
Water.
What you will do:
1. Line up the 3 glasses in a row.
2. Add a few drops of food coloring to glass 1 and a few drops of a different colour to glass 3.
3. Fill glass 1 and glass 3 with water.
4. Fold the paper towels into rectangles and fold in half. Rest half the paper towel in one glass and the other half in the glass next to it.
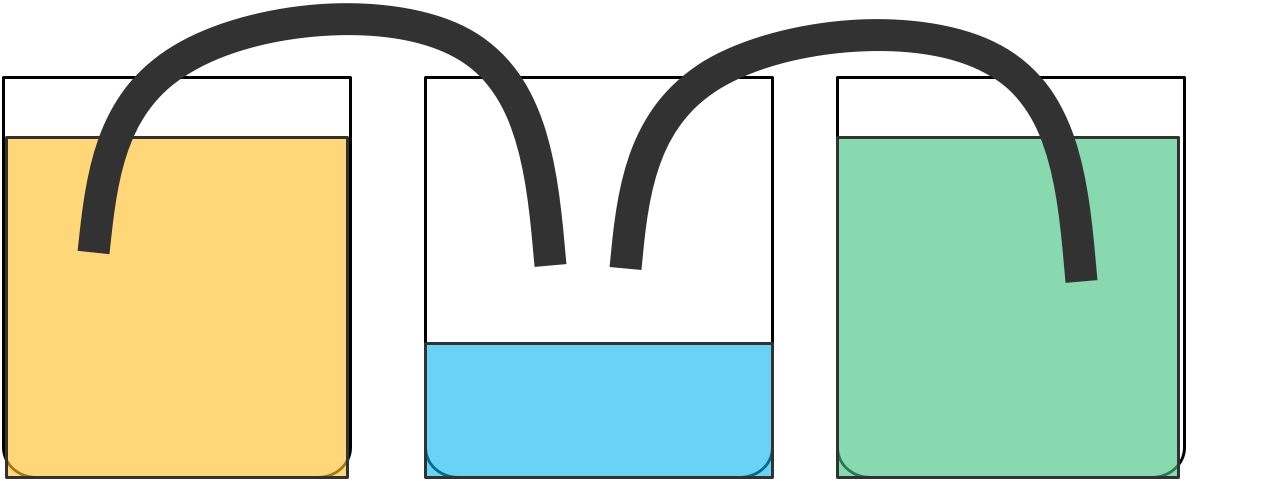
5. Watch the capillary action in progress!
Activity 2
What you need:
Celery or white flowers like carnations
A glass
red or blue food colouring
What you will do:
1. Add a few drops of food colouring to the glass and half fill with water.
2. Remove the end of the stalk from the celery or flower. Stand the celery or flower in the water and watch the capillary action in progress!
-